 Provider News NevadaJuly 1, 2022 July 2022 Anthem Provider News - NevadaPlease note that the important message below from Bryony Winn – President, Health Solutions – applies to our Commercial, Medicaid and Medicare Advantage programs from Anthem Blue Cross and Blue Shield.
I am pleased to announce that our shareholders voted to approve our parent company's name change from Anthem, Inc. to Elevance Health, Inc. (NYSE Ticker Symbol — ELV) effective
June 28, 2022.
Here is what you can expect:
- A bold new vision for the future of health
We chose the name Elevance Health to better reflect our business as we elevate the importance of whole health and advance health beyond healthcare for consumers, their families, and our shared communities. This new vision fuels our transformation from a traditional health benefits organization to a health company that looks beyond the traditional scope of physical health.
- No action is needed by you, and we remain committed to helping you deliver whole-person care for your patients, our customers. Importantly, there is no impact or changes to your contract, reimbursement, or level of support. For your patients, it will not change their plan or coverage or change how they receive their medications. Provider networks will not be changing.
- A more holistic approach to health that improves affordability and outcomes
Bringing together a broad portfolio of health plans, including pharmacy, behavioral, clinical, and complex care provider partners, we can deliver integrated, holistic health solutions to meet the increasing needs of our customers and care provider partners. This includes two notable changes:
- Our healthcare service partners will operate under a new brand called Carelon. This includes Beacon Health Options, AIM Specialty Health®, CareMore, and IngenioRx. You can find us at Carelon.com.
- IngenioRx, our pharmacy benefit management partner, will become CarelonRx on
January 1, 2023. This name change will not impact your patient’s benefits, coverage, or how their medications are filled. We will communicate detailed information about this change soon.
- A simpler brand portfolio that makes it easier to do business with us
We have streamlined and simplified the complexity of our health plan and service businesses and reduced the number of brands we have in the market, so our partners and customers clearly understand where we serve, who we serve, and what our brands do.
What does this mean for care providers?
We will operate as Anthem Blue Cross or Anthem Blue Cross and Blue Shield in our 14 Blue-licensed markets. Our existing Anthem-branded health plans are not changing and will continue to operate in their current states. There will be no impact to plans, coverage, or level of support.
Looking forward together
As your partner, we will continue to keep you updated with new information as soon as it becomes available. In the meantime, you can visit us at ElevanceHealth.com or contact your provider representative with any questions.
Thank you for joining us on this exciting path forward as we reimagine what is possible for every moment of health.
Sincerely,

Bryony Winn
President, Health Solutions
ATTACHMENTS (available on web): Bryony Sig.jpg (jpg - 0.01mb) This communication applies to the Medicaid program from Anthem Blue Cross and Blue Shield Healthcare Solutions and the Medicare Advantage and Commercial programs from
Anthem Blue Cross and Blue Shield (Anthem) in Nevada.
Did you know that Provider Relations has been redesigned into the Provider Experience team?
What’s new?
- Provider Experience works all lines of business: Commercial, Medicaid, and Medicare.
- Provider Experience now focuses on two things:
- Education and training:
- Onboarding for new providers
- Appropriate channels for claim questions
- Provider self-service tools on Availity
- How to submit demographic changes for the provider and/or organization
- Ensuring providers remain up to date about what’s new
- Assisting in issue resolution by:
- Responding to inquiries within 48 to 72 hours
- Increasing focus on understanding and resolving inquiries
- Bettering service across all lines of business to reduce points of contact
- Helping providers when issues are not resolved through standard processes
Need to reach us? Provider Experience has an easy-to-use Contact Us page
Not sure who your Provider Experience consultant is? Don’t worry!
We have a new tool that allows you to submit questions to our team directly at https://providers.anthem.com/nevada-provider/contact-us/email.
Your question will be automatically routed to a Provider Experience consultant, who will assist you within 48 hours.
This communication applies to the Medicare Advantage and Commercial programs from
Anthem Blue Cross and Blue Shield (Anthem) in Nevada.
This is a reminder to ensure that you are referring Anthem members to participating labs. LabCorp is our preferred lab provider and offers a single source solution to your testing needs. The relationship with LabCorp does not affect network hospital-based lab service providers, contracted pathologists, or contracted independent laboratories. Physicians may continue to refer to all par providers as they have in the past.
Not only does your Anthem agreement obligate you to refer to participating labs where available, but members will only receive their full benefits from participating providers. As a result, referring your patient and our member to a non-participating lab may expose them to a greater financial responsibility.
Unfortunately, there are certain non-participating labs that are offering to waive or cap co-payments, coinsurance, or deductibles to our members to increase their overall revenue. These practices undermine member benefits and may encourage over-utilization of services.
These billing practices are also questionable in their legality. Such a practice may present violations under state or federal anti-kickback laws.
For a listing of Anthem participating laboratories, please check our online directory. Go to anthem.com. Choose Select Providers, and Providers Overview. Select Find Resources in Your State and pick Nevada. From the Provider Home tab, select the enter button from the blue box on the left side of page titled Find a Doctor.
Note: When searching for laboratory, pathology, or radiology services, under the field I am looking for a:, select Lab/Pathology/Radiology, and then under the field Who specializes in:, select Laboratories, Pathology, or Radiology as appropriate for your inquiry.
LabCorp is our preferred lab provider and offers a single source solution to your testing needs.
LabCorp provides services that range from routine testing, such as basic blood counts and cholesterol tests, to highly complex diagnosing of genetic conditions, cancers, and other rare diseases. LabCorp has specialized laboratories which cover the following areas of testing:
|
· Allergy program
· Cancer testing
· Cardiovascular disease
· Companion diagnostics
· Dermatology
· Diabetes
· DNA testing
· Endocrine disorders
· Esoteric coagulation
· Gastroenterology
|
· Genetic testing
· Genetic counseling
· Genomics
· Human Leukocyte Antigens (HLA) lab for national marrow donor program
· Hematopathology
· Infectious disease
· Immunology
· Liver disease
· Kidney disease
|
· Medical drug monitoring
· Molecular diagnostics
· Newborn screening
· Pain management
· Pathology expertise with range of subspecialties
· Pharmacogenomics
· Preimplantation genetic diagnosis
· Reproductive health
|
· Obstetrics/ gynecology
· Oncology
· Toxicology
· Whole exome sequencing
· Virology
· Women’s health
· Urology
|
Note: This relationship with LabCorp does not affect network hospital-based lab service providers, or contracted pathologists.
To find a LabCorp location near you, go to www.LabCorp.com or call one of the phone numbers below.
For information about specialized assays or about requirements for special collection kits and specimen handling, call LabCorp at 303-792-2600 or toll free at 888-LABCORP (888-522-2677).
CMS routinely issues revisions to the average sales price (ASP) fee schedules regarding drug pricing. CMS is supplying the third-quarter fee schedule with an effective date of July 1, 2022. This will go into effect with Anthem Blue Cross and Blue Shield (Anthem) on August 1, 2022. To view the ASP fee schedule, please visit the CMS website at http://www.cms.hhs.gov/McrPartBDrugAvgSalesPrice/.
Current provider directory information helps Anthem Blue Cross and Blue Shield members find the most up-to-date information available. As a partner in the care of our members, we ask that you review your online provider directory information regularly and provide updates as needed.
If changes are needed, please take the time to update your information by submitting updates and corrections to us on our online Provider Maintenance Form. Online update options include:
- Adding/changing an address location
- Name change
- Tax ID changes
- Provider leaving a group or a single location
- Phone/fax number changes
- Closing a practice location
Once you submit the Provider Maintenance Form, you will receive an email acknowledging receipt of your request. Visit the Provider Maintenance Form landing page for complete instructions.
The Consolidated Appropriations Act (CAA), effective January 1, 2022, contains a provision that requires online provider directory information be reviewed and updated (if needed) at least every 90 days. Thank you for doing your part in keeping our provider directories current.
We are enhancing our outpatient facility editing to help align with correct coding guidelines for usage of HCPCS code G0463. The code description for G0463 is “hospital outpatient clinic visit or assessment and management of a patient”. Based on this code description, HCPCS code G0463, should only be billed with revenue codes which support the billing of clinic visits/assessment & management services. When G0463 is billed with an inappropriate revenue code, it will be denied. For assistance with coding guidelines, the National Uniform Billing Committee (NUBC) is a valuable resource.
3072F: new language about two-year compliance.
The Comprehensive Diabetes Care HEDIS® measure Retinal Eye Exam (DRE) valuates the percent of adult members ages 18 to 75, with diabetes (type 1 and type 2), who had a retinal eye exam during the measurement year.
Changes to 3072F
The definition for the code 3072F (negative for retinopathy) has been redefined to low risk for retinopathy (no evidence of retinopathy in the prior year). This can be particularly confusing because it would not be used at the time of the exam. It would be used the following year, along with the exam coding for the current year, to indicate that retinopathy was not present the previous year.
A simpler coding solution
Using these three codes count toward the DRE measurement if they are billed in the current measurement year or the prior year. This means you can submit the appropriate code at the time of the exam, and it covers both years:
|
2023F
|
Dilated retinal eye exam with interpretation by an ophthalmologist or optometrist documented and reviewed; without evidence of retinopathy (DM)
|
|
2025F
|
7 standard field stereoscopic retinal photos with interpretation by an ophthalmologist or optometrist documented and reviewed: without evidence of retinopathy (DM)
|
|
2033F
|
Eye imaging validated to match diagnosis from 7 standard field stereoscopic retinal photos results documented and reviewed: without evidence of retinopathy (DM)
|
For more about diabetic retinopathy, visit CMS.gov or use this link to read more.
Meeting the measurement for all diabetes care
These exams are also important in evaluating the overall health of diabetic patients, as well as meeting the Comprehensive Diabetes Care HEDIS measure:
- Hemoglobin A1c (HbA1c) testing
- HbA1c poor control (> 9.0%)
- HbA1c control (< 8.0%)
- Retinal Eye exam performed
- Blood Pressure control (< 140/90 mm Hg)
Record your efforts in the member’s medical records for the HbA1c tests and results, retinal eye exam, blood pressure, urine creatinine test, and the estimated glomerular filtration rate test. Meeting the mark and closing gaps in care is key to good health outcomes.
In a recent study published by Pediatrics, economic hardship, school closing, and shutdowns led to sedentary lifestyles and increases in childhood obesity. The research analyzed doctor visits pre-pandemic then during the pandemic period, and the increases were dramatic. Overall obesity increased from 13.7% to 15.4% in patients 5 to 9 years. Increases from 1% in children aged 13 to 17 to 2.6% for those aged 5 to 9 years were observed.
The study recommended new approaches to Weight Assessment and Counseling. These include recommending virtual activities that promote increased physical activity. Focusing on ways to remain safe and active with outside activities, such as park visits, walks, and bike riding were also suggested.
The Centers for Disease Control and Prevention has a great resource called Ways to Promote Health with Preschoolers. This fun flyer shows how we can all work together to support a healthy lifestyle. You can download a copy here.
The HEDIS® measure Weight Assessment and Counseling for Nutrition and Physical Activity for Children/Adolescents (WCC) requires a nutritional evaluation and pro-active guidance as part of a routine health visit:
- When counseling for nutrition, document current nutritional behavior, such as meal patterns, eating and diet habits, and weight counseling.
- When counseling for physical activity, document current physical activity behavior, such as exercise routine, participation in sports activities, bike riding and play groups.
- Handouts about nutrition and physical activity also count toward meeting this HEDIS measure when documented in the member’s health record.
HEDIS® is a registered trademark of the National Committee for Quality Assurance (NCQA).
HEDIS® measure WCC looks at the percentage of members, 3 to 17 years of age, who had an outpatient visit with a PCP or OB/GYN and have documented evidence for all the following during the measurement year:
- Body mass index (BMI) percentile (percentage, not value).
- Counseling for nutrition.
- Counseling for physical activity.
Telehealth, virtual check-in, and telephone visits all meet the criteria for nutrition and physical activity counseling. Counseling does not need to take place only during a well-visit, WCC can also be completed during sick visits. Documenting guidance in your patient’s records is key.
Code services correctly to measure success.
These diagnosis and procedure codes are used to document BMI percentile, weight assessment, and counseling for nutrition and physical activity:
|
Description
|
CPT®
|
ICD-10-CM
|
HCPCS
|
|
BMI percentile
|
|
Z68.51-Z68.54
|
|
|
Counseling for nutrition
|
97802, 97803,
97804
|
Z71.3
|
G0270, G0271, G0447, S9449,
S9452, S9470
|
|
Counseling for physical activity
|
|
Z02.5, Z71.82
|
G0447, S9451
|
|
Codes to identify outpatient visits:
CPT — 99201-99205, 99211-99215, 99241-99245, 99341-99345, 99347-99350, 99381-99387, 99391-99397, 99401-99404, 99411, 99412, 99429, 99455, 99456, 99483
HCPCS — G0402, G0438, G0439, G0463, T1015
|
|
American Academy of Pediatrics. American Academy of Pediatrics raises concern about children’s nutrition and physical activity during pandemic. Available at: http://services.aap.org/en/news-room/news-releases/aap/2020/american-academy-of-pediatrics-raises-concern-about-childrens-nutrition-and-physical-activity-during-pandemic/. Accessed December 10, 2020.
Submitting attachments electronically is the most efficient way for you to receive your claim payments faster — that’s why we have been hard at work making the digital attachment process easier, more intuitive, and streamlined.
We’re preparing to launch an enhancement to the Claims Status Inquiry application that will enable you to submit claims attachments directly to the claim from Availity.com.
Submitting attachments electronically:
- Reduces costs associated with manual submission.
- Reduces errors associated with matching the claim when attachments are submitted manually.
- Reduces delays in payments.
- Saves time: No need to copy, fax, or mail.
- Reduces the exchange of unnecessary member information and too much personal health information sharing.
If your workflow for attachments is through EDI submissions or directly through the Availity application, we have a solution for that.
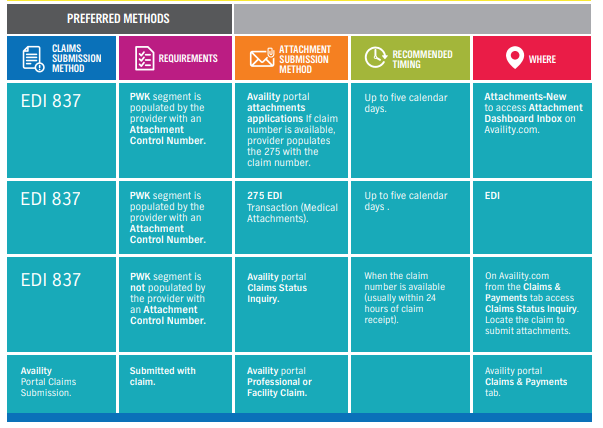
Didn’t submit your attachment with your claim? No problem!
If you submitted your claim through EDI using the 837, and the PWK segment contains the attachment control number, there are three options for submitting attachments:
- Through the attachments dashboard inbox:
- From com, select the Claims & Payments tab to access Attachments – New and your Attachments Dashboard Inbox
- Through the 275 attachment:
- Important: You must populate the PWK segment on the 837 with your document control number to ensure the claim can match to the attachment.
- Through the Availity.com application:
- From com, select the Claims & Payments tab to run a Claims Status Inquiry to locate your claim. When you have found your claim, select the Send Attachments button:
- If you submitted your claim through the Availity application, simply submit your attachment with your claim
- If you need to add additional attachments, to add a forgotten attachment, or for claims adjustments:
- From com, select the Claims & Payments tab and run a Claims Status Inquiry to locate your claim. When you have found your claim, use the Send Attachments button.
For more information and educational webinars
In collaboration with Availity, we will hold a series of educational webinars that includes a deep dive into EDI attachment submissions, as well as the new Claims Status Inquiry workflow. Sign up today.
ATTACHMENTS (available on web): 2397 Image.png (png - 0.09mb) Anthem Blue Cross and Blue Shield is setting up a new digital education platform called the Provider Learning Hub. Initially, the Provider Learning Hub will include how-to instructions for Availity* registration and onboarding. Our first featured training is focused on the Attachment application with special emphasis on new processes that should make submitting attachments more efficient.
You can access the new Provider Learning Hub from the home page on our public website under Important Announcements in mid-July or by navigating to this link: https://gateway.on24.com/wcc/eh/3555851/category/104185/anthem-blue-cross-and-blue-shield.
This communication applies to the Commercial and Medicare Advantage programs from
Anthem Blue Cross and Blue Shield (Anthem).
In an effort to deliver on Anthem’s purpose to improve the health of humanity, we now have a program for in-home patient care for acute conditions.
Anthem’s Hospital in Home program can advise capable, innovative hospital partners in developing their own hospital in home programs. Once implemented, patients can recover in a more comfortable environment, allowing hospitals to keep beds available for patients with more complex needs.
Inpatient level of care in the home can be a welcome alternative to traditional hospital settings. Patients may find acute care at home to be more convenient and less stressful, and studies have shown acute care at home can be safe and allow for smoother transition to self-care management after the acute illness. Hospital in Home clinical trials demonstrate a 25% decrease in readmissions and a 50% reduction in time spent in bed.1
Anthem’s Hospital in Home program has a set of minimum requirements that are designed to promote patient safety. These requirements include aspects of the member’s home environment, the clinical scenario, remote monitoring capabilities, and plans for program evaluation.
Please contact your Anthem contracting representative to learn more about Anthem’s Hospital in Home program.
Effective December 31, 2022, the enhanced reimbursement billing opportunity (S-codes) for medical oncologists selecting on-pathway drug regimens as part of the AIM Specialty Health ®* Medical Oncology Solution/ Cancer Care Quality Program (CCQP) chemotherapy authorization process will be discontinued.
The CCQP S-codes S0353 and S0354 were activated on July 1, 2014, and have supported providers with member care coordination, and adoption of optimal, evidence-based oncology drug regimens.
The CCQP/AIM pathways will continue to enable the delivery of clinically appropriate cancer treatment, and supportive medication that ensures members receive high-quality, patient-centered care. The AIM pathways will remain available to medical oncologists and related subspeciality providers via the AIM provider website.
As the CCQP continues to evolve, the program will become a key component of more comprehensive value-based cancer care improvement initiatives, including the Oncology Medical Home Plus (OMH+) program, launching on July 1, 2022, and January 1, 2023.
Contact your Anthem Blue Cross and Blue Shield network representative or your oncology provider engagement liaison for more information.
Material Adverse Change
Anthem Blue Cross and Blue Shield and our subsidiary company, HMO Nevada (Anthem) are pleased to provide you with our updated and new medical policies. Anthem will also be implementing changes to our Clinical Utilization Management (UM) Guidelines that are adopted for Nevada. The Clinical UM guidelines published on our website represent the clinical UM guidelines currently available to all Plans for adoption throughout our organization. Because local practice patterns, claims systems and benefit designs vary, a local Plan may choose whether or not to implement a particular clinical UM guideline.
The major new policies and changes are summarized below. Please refer to the specific policy for coding, language, and rationale updates and changes that are not summarized below.
New Medical Policies effective for service dates on and after October 1, 2022
DME.00046 Intermittent abdominal pressure ventilation devices
This document addresses the use of intermittent abdominal pressure ventilation devices.
- Considered investigational and not medically necessary for all indications.
- Prior authorization required effective July 1, 2022.
DME.00047 Rehabilitative Devices with Remote Monitoring
This document addresses the use of rehabilitative devices with remote monitoring and adjustment capabilities intended to evaluate and improve muscle strength and range of motion while reporting session data to the individual’s provider.
- Considered investigational and not medically necessary for all indications.
- Prior authorization required effective July 1, 2022.
DME.00048 Virtual Reality-Assisted Therapy Systems
This document addresses the use of virtual reality-assisted therapy systems that may be used in the management of pain, cognitive or motor rehabilitation, treatment of procedural anxiety, and promotion of weight control.
- Considered investigational and not medically necessary for all indications.
- Prior authorization required effective July 1, 2022.
GENE.00059 Hybrid Personalized Molecular Residual Disease Testing for Cancer
This document addresses hybrid personalized molecular residual disease (MRD) testing for oncologic disease management. This personalized testing occurs in a two-step process. The first step involves whole exome sequencing (WES) of the tumor tissue. In the second step, information about the tumor learned from the WES is used to develop a personalized assay to to detect circulating tumor DNA (ctDNA) that assesses MRD. Commercially available personalized MRD tests include the Signatera™ test (Natera Inc., San Carlos, CA) and the RaDaR™ test (Inivata, Research Triangle Park, NC).
- Considered investigational and not medically necessary for all indications.
- Prior authorization required effective July 1, 2022.
LAB.00048 Pain Management Biomarker Analysis
This document addresses a new pain biomarker test, the Foundation Pain Index (FPI) which is a test panel of pain functional biomarkers in urine and is intended to identify sources of chronic pain. The FPI involves analysis of urine by liquid chromatography tandem mass spectrometry (LCM/MS) of a panel of 11 endogenous analytes (methylmalonic acid, xanthurenic acid, homocysteine, pyroglutamic acid, vanilmandelate, 5-hydroxyindoleacetic acid, hydroxymethylglutarate, ethylmalonate, 3-hydroxypropyl mercapturic acid [3-HPMA], quinolinic acid, kynurenic acid). It is suggested that nutritional deficiencies (such as in Vitamin B12 and B6), oxidative stress and metabolic abnormalities can lead to pain syndromes, and that these abnormalities can be identified through this testing for these pain biomarkers.
- Considered investigational and not medically necessary for all indications.
- Prior authorization required effective July 1, 2022.
MED.00139 Electrical Impedance Scanning for Cancer Detection This document addresses the use of electrical impedance scanning for cancer detection.
- Considered investigational and not medically necessary for all indications.
- Prior authorization required effective July 1, 2022.
TRANS.00039 Portable Normothermic Organ Perfusion Systems
This document addresses use of a portable normothermic organ machine perfusion and monitoring medical device used to preserve donor organs in a near-normothermic state from retrieval until transplantation. This document does not address static cold storage or other forms of solid organ preservation.
- Considered medically necessary when used for preservation of donor lung pairs initially deemed unacceptable for procurement and transplantation based on limitations of cold storage preservation, that is: age greater than 55, PaO2/FiO2 less than 300 mmHg, donation after cardiac death (DCD) donors, ischemic time greater than 6 hours).
- Considered medically necessary when used for the preservation of an organ initially deemed unacceptable and when criteria (1 or 2) below are met:
- Organ Care System Liver: Liver allografts from donors after circulatory death (DCD) less than or equal to 55 years old and with less than or equal to 30 minutes of warm ischemic time, macrosteatosis less than or equal to 15%; or
- OrganOx metra System: liver allografts from donors after DCD less than or equal to 40 years of age, with less than or equal to 20 minutes of functional warm ischemic time, and macrosteatosis less than or equal to 15%.
- Considered investigational and not medically necessary when the above criteria are not met, including but not limited to the preservation of other solid donor organs, including the heart (that is, OCS Heart System), or preservation of standard criteria donor organ.
- Prior authorization required effective July 1, 2022.
Revised Medical Policies and Adopted Clinical UM Guidelines effective July 1, 2022:
CG-SURG-82 Bone-Anchored and Bone Conduction Hearing Aids
- Clarified the criteria for unilateral hearing loss to include conductive, mixed and sensorineural hearing loss
- Clarified the MN criteria for replacement parts and upgrades
SURG.00097 Scoliosis Surgery
- Added MN criteria for vertebral body tethering
Revised Medical Policies and Adopted Clinical UM Guidelines effective October 1, 2022:
CG-SURG-61 Cryosurgical, Radiofrequency or Laser Ablation to Treat Solid Tumors Outside the Liver
- Revised title
- Removed the reference to glomerular filtration rate from the radiofrequency and cryosurgical ablation treatment of renal cancer
- Added the term “metastatic” to the radiofrequency ablation treatment of metastatic lung cancer to clarify extra-pulmonary disease
- Added NMN statement for laser ablation therapy
- Removed examples from the cryosurgical and radiofrequency ablation NMN statements
GENE.00023 Gene Expression Profiling of Melanomas and Cutaneous Squamous Cell Carcinoma
- Revised title
- Expanded Scope and Position Statement to include cutaneous squamous cell carcinoma
Medical Policies and Clinical Guideline archived May 19, 2022, except where noted
SURG.00101 Suprachoroidal Injection of a Pharmacologic Agent
Medical Policies and Clinical Guideline archived June 29, 2022, except where noted
MED.00121 Implantable Interstitial Glucose Monitors
- Moved content into CG-DME-42 Continuous Glucose Monitoring Devices and External Insulin Infusion Pumps
Medical Policies and Clinical Guideline archived July 6, 2022, except where noted
DME.00024 Transtympanic Micropressure
SURG.00137 Focused Microwave Thermotherapy for Breast Cancer
MED.00127 Chelation Therapy
- Transitioned to CG-MED-90 Chelation Therapy
Clinical Guidelines de-adopted January 1, 2022
- CG-LAB-11 Screening for Vitamin D Deficiency in Average Risk Individuals
Clinical Guidelines de-adopted February 1, 2022
- CG-MED-63 Treatment of Hyperhidrosis
- CG-MED-69 Inhaled Nitric Oxide
- CG-OR-PR-04 Cranial Remodeling Bands and Helmets (Cranial Orthotics)
- CG-SURG-03 Blepharoplasty, Blepharoptosis Repair, and Brow Lift
- CG-SURG-18 Septoplasty
- CG-SURG-24 Functional Endoscopic Sinus Surgery (FESS)
- CG-SURG-34 Diagnostic Infertility Surgery
- CG-SURG-59 Vena Cava Filters
- CG-SURG-70 Gastric Electrical Stimulation
- CG-SURG-73 Balloon Sinus Ostial Dilation
- CG-SURG-75 Transanal Endoscopic Microsurgical (TEM) Excision of Rectal Lesions
- CG-SURG-84 Mandibular/Maxillary (Orthognathic) Surgery
- CG-SURG-94 Keratoprosthesis
- CG-SURG-104 Intraoperative Neurophysiological Monitoring
- CG-SURG-78 Locoregional and Surgical Techniques for Treating Primary and Metastatic Liver Malignancies
Anthem Medical Policies and Clinical UM Guidelines are developed by our national Medical Policy and Technology Assessment Committee. The Committee, which includes Anthem medical directors and representatives from practicing physician groups, meets quarterly to review current scientific data and clinical developments.
All coverage written or administered by Anthem excludes from coverage, services or supplies that are investigational and/or not medically necessary. A member’s claim may not be eligible for payment if it was determined not to meet medical necessity criteria set in Anthem’s medical policies. Review procedures have been refined to facilitate claim investigation.
Anthem’s Medical Policies and Clinical UM Guidelines are available online
The complete list of our Medical Policies and Clinical UM Guidelines may be accessed at anthem.com, and select Providers. Under the Provider Resources heading, select Policies and Guidelines. Select Nevada as Your State. Select View Medical Policies & UM Guidelines. Either enter kew word or code, or select the link for Full List page to search the policy for your inquiry.
To view the list of specific clinical UM guidelines adopted by Nevada, navigate to the View Medical Policies & UM Guidelines page. Scroll to the bottom of the page to the link titled Clinical UM Guidelines adopted by Anthem Blue Cross and Blue Shield in Nevada.
Material adverse change
Beginning with dates of service on or after October 1, 2022, Anthem Blue Cross and Blue Shield will implement the following:
- 96365, 96369, 96372, 96373, 96374, 96379 will deny when reported with 78265, 78830 or 78835.
- 95957 will deny when reported with 95700 on the same day:
- The reference to subsequent dates of service was removed from this code pair.
For specific policy details, visit the Reimbursement Policy page on the provider website.
Material adverse change
Beginning with dates of service on or after October 1, 2022, Anthem Blue Cross and Blue Shield (Anthem) will update the Modifiers Impacting Adjudication code list to not allow reimbursement for CPT® code 99211 when appended with a modifier 25.
For specific policy details, visit the reimbursement policy page at anthem.com provider website.
Material adverse change
Beginning with dates of service on or after October 1, 2022, the Anthem Blue Cross and Blue Shield Laboratory and Venipuncture Services policy is expanded to include facility providers. The related coding section is updated to clarify coding for professional and facility providers.
Facility providers are not eligible for separate reimbursement for the following select
specimen-handling CPT®/HCPCS codes: 99000, 99001, H0048, P9603, and P9604. In addition, Related Coding section 1 in the Bundled Services and Supplies policy is updated to remove these codes.
For specific policy details, visit the reimbursement policy page at anthem.com provider website.
Material adverse change
Beginning with dates of service on or after October 1, 2022, Anthem Blue Cross and Blue Shield (Anthem) will implement a new professional and facility reimbursement policy titled, Modifier FB — Professional and Facility. Modifier FB should be appended to all devices, supplies, or drugs obtained at no cost to the provider. Services appended with modifier FB are not eligible for reimbursement.
In addition, Modifier FB has been removed from the Modifier Rules — Professional policy.
For specific policy details, visit the reimbursement policy page at anthem.com provider website.
In the September 2021 edition of Provider News, we announced that a new commercial reimbursement policy titled ‘Sexually Transmitted Infections - professional’ would be effective for dates of service on or after December 1, 2021. We have made a decision to retract this reimbursement policy.
Anthem Blue Cross and Blue Shield (Anthem) has added value to the Transitional Care Management definition to include discharge from the emergency room to help prevent future emergency room encounters or hospital admissions.
As of April 27, 2022, Anthem has implemented a new professional reimbursement policy: Transitional Care Management. When a member requires a transition to a community setting, the Transitional Care Management period begins upon the member’s discharge and continues for 29 days.
For specific policy details, visit the Reimbursement Policy page on the provider website.
Material adverse change
Beginning with dates of service on or after October 1, 2022, Anthem Blue Cross and Blue Shield will update the policy language to indicate the following:
- The title of the policy will be renamed to Place of Service – Facility from Place of Service Evaluation and Management Services – Facility.
- Professional services billed under revenue codes 960-983 are nonreimbursable when submitted on a UB-04.
- Preventive Counseling CPTs 99406–99409, 99411, and 99412 are nonreimburseable when billed in an outpatient setting.
As a reminder, Evaluation and Management (E/M) services and other professional services (excluding evaluation and management services rendered in the emergency room and billed with ER revenue codes) are required to be billed on a CMS 1500 form.
For specific policy details, visit the reimbursement policy page at anthem.com provider website.
Visit the Drug Lists page for more information on:
- Copayment/coinsurance requirements and their applicable drug classes.
- Drug lists and changes.
- Prior authorization criteria.
- Procedures for generic substitution.
- Therapeutic interchange.
- Step therapy or other management methods subject to prescribing decisions.
- Any other requirements, restrictions, or limitations that apply to using certain drugs.
The commercial drug list is posted to the website quarterly on the first day of the month in January, April, July, and October.
Federal Employee Program pharmacy updates and other pharmacy related information may be accessed at www.fepblue.org > Pharmacy Benefits.
Medicaid
Please continue to check Medicaid Provider Communications & updates at anthem.com/nvmedicaiddoc for the latest Medicaid information, including:
Medicaid
Providers have asked how they can make requests to the state to add non-covered codes. We have recently created a new process to make these requests:
- Send an email request to Erin Lynch, Chief of the Medical Programs Unit, at erin.lynch@dhcfp.nv.gov:
- Your request should include the codes that are not currently covered as well as the reasons they should be covered. Please also include any supporting documentation.
- After Erin Lynch’s team reviews the request, you may receive a letter inviting you to present your request in front of the Medical Care Advisory Committee (MCAC):
- Your request will be further reviewed, researched, and vetted prior to being brought before the legislative session for consideration and possible budget approval.
If you have questions, please contact Erin Lynch at erin.lynch@dhcp.nv.gov.
Medicaid
This communication applies to the Medicaid program from Anthem Blue Cross and Blue Shield Healthcare Solutions and the Medicare Advantage and Commercial programs from
Anthem Blue Cross and Blue Shield (Anthem) in Nevada.
Did you know that Provider Relations has been redesigned into the Provider Experience team?
What’s new?
- Provider Experience works all lines of business: Commercial, Medicaid, and Medicare.
- Provider Experience now focuses on two things:
- Education and training:
- Onboarding for new providers
- Appropriate channels for claim questions
- Provider self-service tools on Availity
- How to submit demographic changes for the provider and/or organization
- Ensuring providers remain up to date about what’s new
- Assisting in issue resolution by:
- Responding to inquiries within 48 to 72 hours
- Increasing focus on understanding and resolving inquiries
- Bettering service across all lines of business to reduce points of contact
- Helping providers when issues are not resolved through standard processes
Need to reach us? Provider Experience has an easy-to-use Contact Us page
Not sure who your Provider Experience consultant is? Don’t worry!
We have a new tool that allows you to submit questions to our team directly at https://providers.anthem.com/nevada-provider/contact-us/email.
Your question will be automatically routed to a Provider Experience consultant, who will assist you within 48 hours.
Medicaid
Effective August 1, 2018, presumptive drug screens are limited to one per day with a maximum of 20 tests per 12-rolling months. Only three definitive drug screens are permitted per recipient per 12-rolling months. Should more than 20 presumptive screens or more than three definitive screens be needed in 12-rolling months, a prior authorization is required. Claims paid in excess of these limits, without an approved prior authorization, are subject to recoupment.
The impacted provider types (PTs) are: 12 (Hospital, outpatient), 17 (Special clinics), 20 (Physician, M.D., Osteopath, D.O.), 24 (Advanced practice registered nurse), 43 (Laboratory, pathology), 60 (School based), 74 (Nurse midwife) and 77 (Physician’s assistant).
Medicaid Services Manual (MSM) Chapter 800 Laboratory Services has been updated with the following information:
Section 803.1A coverage and limitations
Drug screening and testing:
- Drugs or drug classes for which screening is performed should only reflect those likely to be present based on the recipient’s medical history, current clinical presentation, or risk potential for abuse and diversion.
- Each drug or drug class being tested for must be indicated by the referring physician in a written order and reflected in the patient’s medical record. This information must be patient-specific and accurately reflect the need for each test and must include the specific drugs being screened including recipient diagnosis.
- Current coding for testing of drugs relies on a structure of screening (known as presumptive screening) and may be followed by quantitative measurements (known as definitive testing) that identifies the specific drug or drugs and quantity in the recipient:
- Only one presumptive test performed by direct observation or instrument assisted direct observation or instrument chemistry analyzers may be billed per recipient per day within a maximum of 20 presumptive test per 12-rolling months.
- If the recipient should require more than 20 presumptive tests per 12-rolling months, a prior authorization is required.
- Only three definitive drug tests are permitted per recipient per 12-rolling months.
- Definitive testing is only covered to confirm an unexpected result or identify drugs or metabolites that cannot be detected on a presumptive drug screen.
- Definitive testing should be based on the recipient’s presentation and history and only include what is needed for safe pain management.
- Standing orders for presumptive drug screens may be utilized but must be individualized for each member, signed, and dated by the treating practitioner and updated every 30 days. Standing orders are not permitted for definitive drug screens. Web announcement 1713 October 18, 2018.
- Procedure codes should be reported with a quantity of one per episode of care, regardless of the number of collection/testing items used, the number of procedures, and/or the drug classes screened.
- Testing for the same drug with a blood and urine specimen simultaneously is not covered.
- Drug screening for pre-employment or employment purposes, medicolegal, and/or court ordered that do not meet medical necessity and/or drug screenings for participation in school or military are not covered.
- Routine drug screening is not covered unless used in conjunction with an extended course of treatment for substance use disorders. Specific intervals, at which recipient test should be performed, based on their individual needs, must be documented in the member’s medical record with their treatment plan.
- Drug confirmation tests are not eligible to be separately reported under any procedure code, unlisted, or otherwise
Information follows web announcement 1713 from the State of NV and can be found in the Medicaid Service Manual (MSM) Chapter 800 Laboratory Services: https://dhcfp.nv.gov/Resources/AdminSupport/Manuals/MSM/C800/Chapter800
If you have questions about this communication, contact your Provider Services at 844-396-2330 or use the Contact Us at the bottom of our provider website (https://providers.anthem.com/nv) for up-to-date contact information.
Medicaid
The Medical Policies, Clinical Utilization Management (UM) Guidelines and Third-Party Criteria below were developed and/or revised to support clinical coding edits. Note, several policies and guidelines were revised to provide clarification only and are not included. Existing precertification requirements have not changed.
Please share this notice with other members of your practice and office staff.
To view a guideline, visit https://www.anthem.com/provider/policies/clinical-guidelines/search.
Notes/updates:
Updates marked with an asterisk (*) notate that the criteria may be perceived as more restrictive:
- *CG-LAB-20 — Thyroid Testing:
- Outlines the Medically Necessary and Not Medically Necessary criteria for thyroid testing
- *CG-LAB-21 — Serum Iron Testing:
- Outlines the Medically Necessary and Not Medically Necessary criteria for serum iron testing
- *LAB.00043 — Immune Biomarker Tests for Cancer:
- Oncologic immune biomarker tests are considered Investigational and Not Medically Necessary for all indications
- *LAB.00044 — Saliva-Based Testing to Determine Drug-Metabolizer Status:
- Saliva-based testing to determine drug-metabolizer status is considered Investigational and Not Medically Necessary for all indications
- *LAB.00045 — Selected Tests for the Evaluation and Management of Infertility:
- The following tests or procedures are considered Investigational and Not Medically Necessary for diagnosing or managing infertility:
- Endometrial receptivity analysis
- Sperm-capacitation test
- Sperm deoxyribonucleic acid (DNA) fragmentation test
- Sperm penetration assay
- Uterine natural killer (uNK) cells test
- *LAB.00046 — Testing for Biochemical Markers for Alzheimer’s Disease:
- Measurements of biochemical markers (including but not limited to tau protein, AB-42, neural thread protein) is considered Investigational and Not Medically Necessary as a diagnostic technique for individuals with symptoms suggestive of Alzheimer’s disease
- Measurements of biochemical markers as a screening technique in asymptomatic individuals with or without a family history of Alzheimer’s disease is considered Investigational and Not Medically Necessary
- Moved content related to biomarker testing for Alzheimer’s disease from GENE.00003 Biochemical Markers for the Diagnosis and Screening of Alzheimer’s Disease to this document
- *RAD.00067 — Quantitative Ultrasound for Tissue Characterization:
- Quantitative ultrasound for tissue characterization is considered Investigational and Not Medically Necessary for all indications
- *SURG.00154 — Microsurgical Procedures for the Prevention or Treatment of Lymphedema:
- Revised Position Statement to include the prevention of lymphedema
- *SURG.00160 — Implanted Port Delivery Systems to Treat Ocular Disease:
- The use of a port delivery system to treat ocular disease is considered Investigational and Not Medically Necessary for all indications
- *TRANS.00038 — Thymus Tissue Transplantation:
- Outlines the Medically Necessary and Investigational and Not Medically Necessary criteria for allogeneic processed thymus tissue
Effective June 11, 2022, Anthem Blue Cross and Blue Shield Healthcare Solutions (Anthem) will begin using the AIM Specialty Health®1 Clinical Appropriateness Guidelines for medical necessity review of the below services. Please note, the Anthem Utilization Management team will complete these reviews using the AIM Clinical Appropriateness Guidelines:
- Musculoskeletal guidelines
- Spine surgery
- Joint surgery
- Small joint surgery
- Sacroiliac joint fusion
- Sleep disorder management guideline
- Rehabilitative services
- Occupational therapy
- Physical therapy
- Speech therapy
Medical Policies
On February 17, 2022, the Medical Policy and Technology Assessment Committee (MPTAC) approved the following Medical Policies applicable to Anthem. These guidelines take effect
June 11, 2022.
|
Publish date
|
Medical policy number
|
Medical Policy title
|
New or revised
|
|
04/13/2022
|
*LAB.00043
|
Immune Biomarker Tests for Cancer
|
New
|
|
04/13/2022
|
*LAB.00044
|
Saliva-based Testing to Determine
Drug-Metabolizer Status
|
New
|
|
04/13/2022
|
*LAB.00045
|
Selected Tests for the Evaluation and Management of Infertility
|
New
|
|
04/13/2022
|
*LAB.00046
|
Testing for Biochemical Markers for Alzheimer’s Disease
|
New
|
|
04/13/2022
|
*RAD.00067
|
Quantitative Ultrasound for Tissue Characterization
|
New
|
|
04/13/2022
|
*SURG.00160
|
Implanted Port Delivery Systems to Treat Ocular Disease
|
New
|
|
03/25/2022
|
*TRANS.00038
|
Thymus Tissue Transplantation
|
New
|
|
04/13/2022
|
GENE.00052
|
Whole Genome Sequencing, Whole Exome Sequencing, Gene Panels, and Molecular Profiling
|
Revised
|
|
04/1/2022
|
SURG.00011
|
Allogeneic, Xenographic, Synthetic, Bioengineered, and Composite Products for Wound Healing and Soft Tissue Grafting
|
Revised
|
|
02/24/2022
|
SURG.00036
|
Fetal Surgery for Prenatally Diagnosed Malformations
|
Revised
|
|
04/13/2022
|
SURG.00096
|
Surgical and Ablative Treatments for Chronic Headaches
|
Revised
|
|
04/13/2022
|
*SURG.00154
|
Microsurgical Procedures for the Prevention or Treatment of Lymphedema
|
Revised
|
Clinical UM Guidelines
On February 17, 2022, the MPTAC approved the following Clinical UM Guidelines applicable to Anthem. These guidelines were adopted by the medical operations committee for Nevada Medicaid members on March 24, 2022. These guidelines take effect June 11, 2022.
|
Publish date
|
Clinical UM Guideline number
|
Clinical UM Guideline title
|
New or revised
|
|
04/13/2022
|
*CG-LAB-20
|
Thyroid Testing
|
New
|
|
04/13/2022
|
*CG-LAB-21
|
Serum Iron Testing
|
New
|
|
04/13/2022
|
CG-ANC-03
|
Acupuncture
|
Revised
|
|
04/13/2022
|
CG-GENE-14
|
Gene Mutation Testing for Cancer Susceptibility and Management
|
Revised
|
|
04/13/2022
|
CG-MED-73
|
Hyperbaric Oxygen Therapy (Systemic/Topical)
|
Revised
|
|
04/13/2022
|
CG-SURG-36
|
Adenoidectomy
|
Revised
|
|
02/24/2022
|
CG-SURG-86
|
Endovascular/Endoluminal Repair of Aortic Aneurysms, Aortoiliac Disease, Aortic Dissection and Aortic Transection
|
Revised
|
Medicaid
On November 19, 2021, January 4, 2022, and February 25, 2022, the Pharmacy and Therapeutics (P&T) Committee approved the following Clinical Criteria applicable to the medical drug benefit for Anthem Blue Cross and Blue Shield Healthcare Solutions. These policies were developed, revised, or reviewed to support clinical coding edits.
Visit Clinical Criteria to search for specific policies. If you have questions or would like additional information, use this email.
Please see the explanation/definition for each category of Clinical Criteria below:
- New: newly published criteria
- Revised: addition or removal of medical necessity requirements, new document number
- Updates marked with an asterisk (*) notate that the criteria may be perceived as more restrictive
Please share this notice with other members of your practice and office staff.
Note: The Clinical Criteria listed below applies only to the medical drug benefits contained within the member’s medical policy. This does not apply to pharmacy services.
|
Effective date
|
Document number
|
Clinical Criteria title
|
New or revised
|
|
June 11, 2022
|
*ING-CC-0211
|
Kimmtrak (tebentafusp-tebn)
|
New
|
|
June 11, 2022
|
*ING-CC-0210
|
Enjaymo (sutimlimab-jome)
|
New
|
|
June 11, 2022
|
*ING-CC-0213
|
Voxzogo (vosoritide)
|
New
|
|
June 11, 2022
|
*ING-CC-0212
|
Tezspire (tezepelumab-ekko)
|
New
|
|
June 11, 2022
|
*ING-CC-0086
|
Spravato (esketamine) Nasal Spray
|
Revised
|
|
June 11, 2022
|
ING-CC-0157
|
Padcev (enfortumab vedotin)
|
Revised
|
|
June 11, 2022
|
ING-CC-0125
|
Opdivo (nivolumab)
|
Revised
|
|
June 11, 2022
|
ING-CC-0119
|
Yervoy (ipilimumab)
|
Revised
|
|
June 11, 2022
|
*ING-CC-0099
|
Abraxane (paclitaxel, protein bound)
|
Revised
|
|
June 11, 2022
|
ING-CC-0120
|
Kyprolis (carfilzomib)
|
Revised
|
|
June 11, 2022
|
ING-CC-0126
|
Blincyto (blinatumomab)
|
Revised
|
|
June 11, 2022
|
ING-CC-0129
|
Bavencio (avelumab)
|
Revised
|
|
June 11, 2022
|
*ING-CC-0090
|
Ixempra (ixabepilone)
|
Revised
|
|
June 11, 2022
|
ING-CC-0110
|
Perjeta (pertuzumab)
|
Revised
|
|
June 11, 2022
|
ING-CC-0115
|
Kadcyla (ado-trastuzumab)
|
Revised
|
|
June 11, 2022
|
ING-CC-0108
|
Halaven (eribulin)
|
Revised
|
|
June 11, 2022
|
*ING-CC-0033
|
Xolair (omalizumab)
|
Revised
|
|
June 11, 2022
|
*ING-CC-0043
|
Monoclonal Antibodies to Interleukin-5
|
Revised
|
|
June 11, 2022
|
ING-CC-0038
|
Human Parathyroid Hormone Agents
|
Revised
|
|
June 11, 2022
|
*ING-CC-0186
|
Margenza (margetuximab-cmkb)
|
Revised
|
|
June 11, 2022
|
*ING-CC-0124
|
Keytruda (pembrolizumab)
|
Revised
|
|
June 11, 2022
|
*ING-CC-0078
|
Orencia (abatacept)
|
Revised
|
|
June 11, 2022
|
ING-CC-0050
|
Monoclonal Antibodies to Interleukin-23
|
Revised
|
|
June 11, 2022
|
ING-CC-0042
|
Monoclonal Antibodies to Interleukin-17
|
Revised
|
|
June 11, 2022
|
*ING-CC-0029
|
Dupixent (dupilumab)
|
Revised
|
|
June 11, 2022
|
*ING-CC-0208
|
Adbry (tralokinumab)
|
Revised
|
|
June 11, 2022
|
*ING-CC-0209
|
Leqvio (inclisiran)
|
Revised
|
|
June 11, 2022
|
*ING-CC-0166
|
Trastuzumab Agents
|
Revised
|
|
June 11, 2022
|
*ING-CC-0107
|
Bevacizumab for Non-ophthalmologic Indications
|
Revised
|
Medicare Advantage
Beginning with claims processed on or after August 1, 2022, Anthem Blue Cross and Blue Shield will implement additional steps to review claims for evaluation and management (E/M) services submitted by professional providers when a preventive service (CPT® codes 99381 to 99397) is billed with a problem-oriented E/M service (CPT codes 99202 to 99215) and appended with Modifier 25 (for example, CPT code 99393 billed with CPT code 99213 to 99225).
According to the American Medical Association (AMA) CPT Guidelines, E/M services must be “significant and separately identifiable” in order to appropriately append Modifier 25. Based upon review of the submitted claim information, if the problem-oriented E/M service is determined not to be a significant, separately identifiable service from the preventive service, the problem-oriented E/M service will be bundled with the preventive service.
Providers that believe their medical record documentation supports a significant and separately identifiable E/M service should follow the Claims Payment Dispute process (including submission of such with the dispute) as outlined in the provider manual.
If you have questions on this program, contact your contract manager or Provider Experience.
Medicare Advantage
This communication applies to the Medicare Advantage and Commercial programs from
Anthem Blue Cross and Blue Shield (Anthem) in Nevada.
This is a reminder to ensure that you are referring Anthem members to participating labs. LabCorp is our preferred lab provider and offers a single source solution to your testing needs. The relationship with LabCorp does not affect network hospital-based lab service providers, contracted pathologists, or contracted independent laboratories. Physicians may continue to refer to all par providers as they have in the past.
Not only does your Anthem agreement obligate you to refer to participating labs where available, but members will only receive their full benefits from participating providers. As a result, referring your patient and our member to a non-participating lab may expose them to a greater financial responsibility.
Unfortunately, there are certain non-participating labs that are offering to waive or cap co-payments, coinsurance, or deductibles to our members to increase their overall revenue. These practices undermine member benefits and may encourage over-utilization of services.
These billing practices are also questionable in their legality. Such a practice may present violations under state or federal anti-kickback laws.
For a listing of Anthem participating laboratories, please check our online directory. Go to anthem.com. Choose Select Providers, and Providers Overview. Select Find Resources in Your State and pick Nevada. From the Provider Home tab, select the enter button from the blue box on the left side of page titled Find a Doctor.
Note: When searching for laboratory, pathology, or radiology services, under the field I am looking for a:, select Lab/Pathology/Radiology, and then under the field Who specializes in:, select Laboratories, Pathology, or Radiology as appropriate for your inquiry.
LabCorp is our preferred lab provider and offers a single source solution to your testing needs.
LabCorp provides services that range from routine testing, such as basic blood counts and cholesterol tests, to highly complex diagnosing of genetic conditions, cancers, and other rare diseases. LabCorp has specialized laboratories which cover the following areas of testing:
|
· Allergy program
· Cancer testing
· Cardiovascular disease
· Companion diagnostics
· Dermatology
· Diabetes
· DNA testing
· Endocrine disorders
· Esoteric coagulation
· Gastroenterology
|
· Genetic testing
· Genetic counseling
· Genomics
· Human Leukocyte Antigens (HLA) lab for national marrow donor program
· Hematopathology
· Infectious disease
· Immunology
· Liver disease
· Kidney disease
|
· Medical drug monitoring
· Molecular diagnostics
· Newborn screening
· Pain management
· Pathology expertise with range of subspecialties
· Pharmacogenomics
· Preimplantation genetic diagnosis
· Reproductive health
|
· Obstetrics/ gynecology
· Oncology
· Toxicology
· Whole exome sequencing
· Virology
· Women’s health
· Urology
|
Note: This relationship with LabCorp does not affect network hospital-based lab service providers, or contracted pathologists.
To find a LabCorp location near you, go to www.LabCorp.com or call one of the phone numbers below.
For information about specialized assays or about requirements for special collection kits and specimen handling, call LabCorp at 303-792-2600 or toll free at 888-LABCORP (888-522-2677).
Medicare Advantage
This communication applies to the Commercial and Medicare Advantage programs from
Anthem Blue Cross and Blue Shield (Anthem).
In an effort to deliver on Anthem’s purpose to improve the health of humanity, we now have a program for in-home patient care for acute conditions.
Anthem’s Hospital in Home program can advise capable, innovative hospital partners in developing their own hospital in home programs. Once implemented, patients can recover in a more comfortable environment, allowing hospitals to keep beds available for patients with more complex needs.
Inpatient level of care in the home can be a welcome alternative to traditional hospital settings. Patients may find acute care at home to be more convenient and less stressful, and studies have shown acute care at home can be safe and allow for smoother transition to self-care management after the acute illness. Hospital in Home clinical trials demonstrate a 25% decrease in readmissions and a 50% reduction in time spent in bed.1
Anthem’s Hospital in Home program has a set of minimum requirements that are designed to promote patient safety. These requirements include aspects of the member’s home environment, the clinical scenario, remote monitoring capabilities, and plans for program evaluation.
Please contact your Anthem contracting representative to learn more about Anthem’s Hospital in Home program.
Medicare Advantage
The Cancer Care Navigator (CCN) program is a comprehensive cancer support solution for oncologists and Anthem Blue Cross and Blue Shield (Anthem) members who are at high risk for complications during treatment. This program is aimed at helping to simplify the complexities of cancer care for members.
Practices are given a single point of contact to connect the practice to the right people at Anthem to help lessen administrative burdens. CCN also gives the practice access to Anthem’s advanced predictive analytics to help identify patients at high risk for complications, in turn allowing providers the opportunity to take preventive action and guide targeted interventions.
Patients are provided with a wealth of support through supplemental services (dietitians, pharmacists, etc.), medication adherence assistance, individualized care plans, and goal setting, as well as after-hours telephonic and digital support.
CCN is the ultimate support service to improve the care experience and quality of life to allow patients time to focus on overall health and well-being. Please feel free to reach out to the CCN team at 866-649-0669.
|


